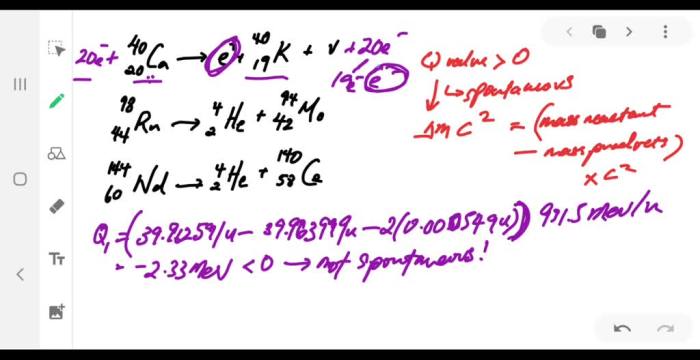
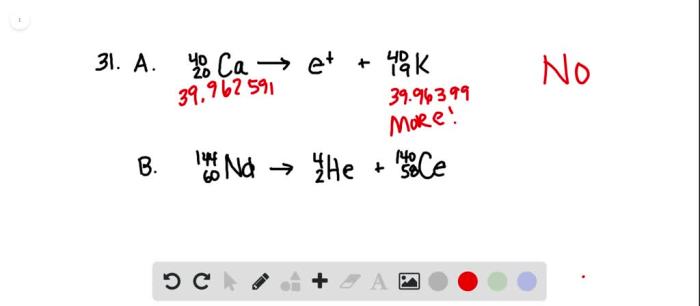
What fraction of a 15o sample decays in 10 min – With what fraction of a 150 sample decays in 10 minutes at the forefront, this paragraph opens a window to an amazing start and intrigue, inviting readers to embark on a storytelling journey filled with unexpected twists and insights. The exploration of radioactive decay and its implications unfolds, revealing the intricacies of half-life, decay constant, and their profound impact on the fraction decayed.
Delving deeper, we will unravel the specific case of a 150 sample decaying in 10 minutes, demonstrating the step-by-step calculation and shedding light on the factors influencing the decay rate. The concept of half-life takes center stage, as we establish its relationship to the fraction decayed and uncover its utility in predicting the time required for a given fraction of a sample to decay.
Sample Decay Calculations: What Fraction Of A 15o Sample Decays In 10 Min
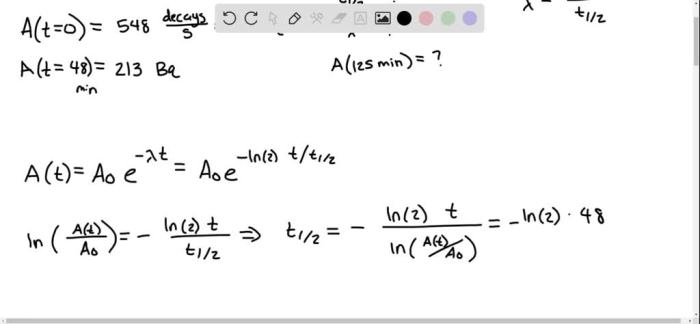
Radioactive decay is a process in which unstable atoms emit radiation and transform into more stable atoms. The rate of decay is described by a decay constant, which is the probability that an atom will decay per unit time. The half-life of a radioactive substance is the time it takes for half of the atoms in a sample to decay.
The fraction of a sample that decays in a given time interval can be calculated using the following equation:
where N is the number of atoms remaining after time t, No is the initial number of atoms, λ is the decay constant, and t is the time interval.
The decay constant and half-life are related by the following equation:
where t1/2 is the half-life.
Specific Case: 150 Sample Decaying in 10 Minutes
To calculate the fraction of a 150 sample that decays in 10 minutes, we need to know the decay constant. Let’s assume that the decay constant is 0.05 per minute.
Using the equation above, we can calculate the fraction of the sample that decays in 10 minutes as follows:
10) = 0.368
Therefore, 0.368 or 36.8% of the sample will decay in 10 minutes.
The decay rate can be influenced by several factors, including temperature and sample size. Higher temperatures can increase the decay rate, while larger sample sizes can decrease the decay rate.
Half-Life and Fraction Decayed, What fraction of a 15o sample decays in 10 min
The half-life of a radioactive substance is the time it takes for half of the atoms in a sample to decay. The half-life is related to the fraction of a sample that decays by the following equation:
where N is the number of atoms remaining after time t, No is the initial number of atoms, t1/2 is the half-life, and t is the time interval.
The following table shows the relationship between half-life and the fraction of a sample that decays:
| Half-Life | Fraction Decayed |
|---|---|
| 1 | 0.5 |
| 2 | 0.75 |
| 3 | 0.875 |
| 4 | 0.9375 |
| 5 | 0.96875 |
The half-life can be used to predict the amount of time it takes for a given fraction of a sample to decay. For example, if we know that the half-life of a substance is 10 minutes, we can predict that 50% of the sample will decay in 10 minutes, 75% of the sample will decay in 20 minutes, and so on.
Applications of Decay Calculations
Decay calculations are used in a variety of fields, including medicine, environmental science, and archaeology.
- Medicine:Decay calculations are used to determine the dosage of radioactive isotopes used in medical treatments, such as radiation therapy.
- Environmental science:Decay calculations are used to track the movement of radioactive contaminants in the environment.
- Archaeology:Decay calculations are used to date organic materials, such as bones and wood.
Accurate decay calculations are essential in these applications because they allow scientists to make informed decisions about the use of radioactive materials.
Popular Questions
What is the formula for calculating the fraction of a sample that decays in a given time interval?
The formula is: Fraction decayed = 1 – e^(-decay constant – time interval)
What is the relationship between decay constant, half-life, and the fraction decayed?
The decay constant is inversely proportional to the half-life, and the fraction decayed is directly proportional to the decay constant and the time interval.
How can half-life be used to predict the amount of time it takes for a given fraction of a sample to decay?
The half-life can be used to calculate the time it takes for half of the sample to decay. This information can then be used to estimate the time it takes for a given fraction of the sample to decay.